Understanding Carbon Atom Electrons: A Deep Dive Into Its Structure And Importance
The carbon atom is a fundamental building block of life, and understanding its electrons is crucial for anyone interested in chemistry and biology. Carbon's unique properties arise from its atomic structure, specifically the arrangement of its electrons. This article will explore the intricacies of carbon atom electrons, their arrangement, and their significance in various chemical processes. By the end of this article, you will have a comprehensive understanding of what makes carbon so essential in the universe.
Carbon is the sixth element on the periodic table, with an atomic number of six. This means that a neutral carbon atom has six protons and six electrons. The arrangement of these electrons determines how carbon interacts with other elements, forming the basis for organic chemistry and life as we know it. In this article, we will delve into the electron configuration of carbon, the role of its electrons in bonding, and the implications of carbon's electron behavior in various scientific fields.
From its role in organic compounds to its significance in biochemical processes, the electrons of carbon atoms are pivotal in understanding not just chemistry, but also biology and environmental science. This article is structured to provide a detailed exploration of carbon atom electrons, their properties, and their applications across different fields. Let’s begin our journey into the world of carbon!
Table of Contents
- 1. Overview of Carbon Atom Structure
- 2. Electron Configuration of Carbon
- 3. The Importance of Electrons in Chemical Bonding
- 4. Types of Chemical Bonds Involving Carbon Electrons
- 5. Carbon in Organic Chemistry
- 6. Carbon Electrons and Biological Processes
- 7. The Role of Carbon in Environmental Science
- 8. Future Research and Applications of Carbon Electrons
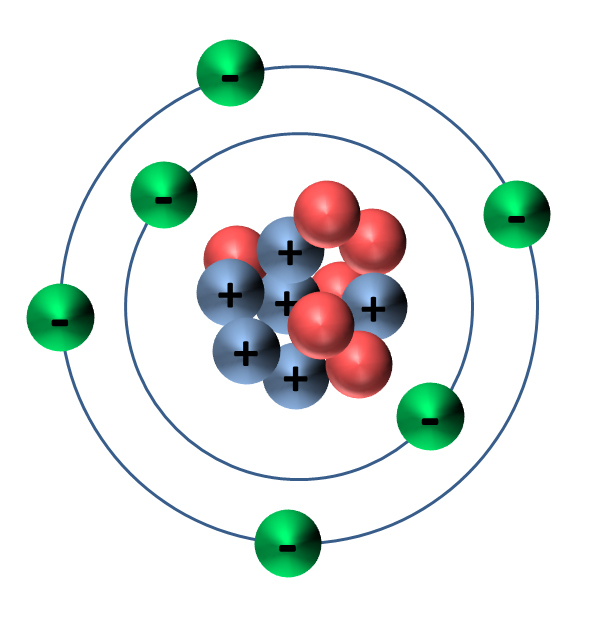
1. Overview of Carbon Atom Structure
The carbon atom is composed of a nucleus containing protons and neutrons, surrounded by electrons that occupy different energy levels or shells. The structure of the carbon atom is fundamental to its properties and behavior in various chemical reactions.
- Atomic Number: 6
- Protons: 6
- Neutrons: 6 (most common isotope)
- Electrons: 6
2. Electron Configuration of Carbon
Carbon's electron configuration is represented as 1s² 2s² 2p². This notation indicates that carbon has two electrons in the first shell (1s) and four electrons in the second shell (two in the 2s and two in the 2p sub-shells). The distribution of these electrons is crucial for understanding how carbon forms bonds with other elements.
2.1 The Significance of Electron Shells
Each electron shell can hold a specific number of electrons, and the configuration determines the atom's reactivity and bonding capacity. Carbon, with four valence electrons, has unique bonding characteristics that allow it to form stable bonds with various elements.
2.2 Valence Electrons and Reactivity
Valence electrons are the outermost electrons and are crucial in chemical bonding. In the case of carbon, the four valence electrons enable it to form up to four covalent bonds with other atoms, making it incredibly versatile in forming complex molecules.
3. The Importance of Electrons in Chemical Bonding
Electrons play a pivotal role in the formation of chemical bonds. The interaction of electrons between atoms leads to various types of bonding, including ionic and covalent bonds. Understanding these interactions is essential for grasping the fundamentals of chemistry.
3.1 Covalent Bonds
Covalent bonds are formed when two atoms share electrons. Carbon's ability to share its four valence electrons allows it to bond with other nonmetals, leading to the formation of a wide range of organic compounds.
3.2 Ionic Bonds
While carbon primarily forms covalent bonds, it can also engage in ionic bonding under certain conditions. This occurs when carbon interacts with highly electronegative elements, leading to the transfer of electrons and the formation of charged ions.
4. Types of Chemical Bonds Involving Carbon Electrons
Carbon can form several types of bonds based on its electron configuration and the nature of the atoms it interacts with. Understanding these bonds is crucial for studying organic and inorganic chemistry.
- Covalent Bonds: Sharing of electrons between atoms.
- Ionic Bonds: Transfer of electrons leading to the formation of charged ions.
- Metallic Bonds: Involves a sea of electrons shared among a lattice of metal atoms.
5. Carbon in Organic Chemistry
Organic chemistry is the study of carbon-containing compounds, and carbon's electron configuration plays a crucial role in the formation of these compounds. The versatility of carbon allows it to form long chains and complex structures, making it the backbone of life.
5.1 Hydrocarbons
Hydrocarbons are organic compounds composed solely of carbon and hydrogen. The electron arrangement in carbon allows it to bond with hydrogen atoms, resulting in a variety of hydrocarbon structures.
5.2 Functional Groups
Carbon can also combine with other elements to form functional groups, which significantly affect the properties and reactions of organic molecules. Understanding these groups is essential for predicting chemical behavior.
6. Carbon Electrons and Biological Processes
Carbon is a key element in biological molecules, such as carbohydrates, proteins, lipids, and nucleic acids. The behavior of carbon electrons directly influences the structure and function of these biomolecules.
6.1 Photosynthesis
In photosynthesis, carbon dioxide is converted into glucose through a series of reactions that involve the transfer and sharing of electrons. This process is fundamental for life on Earth, as it provides the energy needed for growth and metabolism.
6.2 Cellular Respiration
During cellular respiration, the electrons from glucose are transferred to oxygen, releasing energy stored in the chemical bonds. This energy is essential for cellular functions and overall metabolism.
7. The Role of Carbon in Environmental Science
Carbon plays a significant role in environmental science, particularly in understanding climate change and global warming. The behavior of carbon in different forms, such as carbon dioxide and methane, affects atmospheric conditions and climate.
7.1 Carbon Cycle
The carbon cycle describes the movement of carbon through the Earth's systems, including the atmosphere, oceans, and living organisms. Understanding this cycle is crucial for addressing environmental issues.
7.2 Greenhouse Gases
Carbon dioxide and methane are significant greenhouse gases that contribute to the greenhouse effect. The role of carbon in these gases highlights the importance of managing carbon emissions to mitigate climate change.
8. Future Research and Applications of Carbon Electrons
Research on carbon and its electrons continues to evolve, with implications for various fields, including nanotechnology, renewable energy, and materials science. Understanding the behavior of carbon electrons can lead to innovative applications and solutions for global challenges.
8.1 Nanotechnology
Carbon-based nanomaterials, such as graphene and carbon nanotubes, have unique properties due to their electron configurations. These materials are being explored for various applications, from electronics to medicine.
8.2 Renewable Energy
Research into carbon capture and storage technologies aims to reduce carbon emissions and combat climate change. Innovations in this area may lead to more sustainable energy solutions for the future.
Conclusion
In conclusion, understanding carbon atom electrons is essential for grasping the fundamental principles of chemistry, biology, and environmental science. Carbon's unique electron configuration allows it to form diverse compounds and play a crucial role in various processes. As we continue to explore the significance of carbon, we can appreciate its impact on life, technology, and environmental sustainability. If you found this article informative, feel free to leave a comment, share it with others, or explore more articles on our site!
Penutup
Thank you for taking the time to read our comprehensive article on carbon atom electrons. We hope you found it valuable and engaging. Stay tuned for more insightful articles that delve into the fascinating world of science and beyond. Your curiosity is what keeps knowledge alive, and we invite you to return for more explorations in the future!
You Also Like
Understanding The Meaning Of "Down The Rabbit Hole"Exploring The Wing Bucket: A Comprehensive Guide To The Ultimate Dining Experience
A Comprehensive Guide To Australian Shepherd And Collie Mix Dogs
What Are Enzymes And Why Are They Required In Life?
Edwin Sarkissian: The Master Of Visual Storytelling